
褚雪强 副教授 硕士生导师
南京工业大学-化学与分子工程学院-化学楼-B619
Email: xueqiangchu@njtech.edu.cn; ORCID: 0000-0002-9173-1508;
主页:http://chem.njtech.edu.cn/info/1073/3152.htm
每年招收硕士研究生5-12名欢迎联系!
个人小结:
褚雪强,副教授在多氟和全氟烷基化合物(PFASs)的多重C(sp3)-F键官能化反应研究方面取得了具有重要影响力的原创性研究成果,先后在国内外有影响的学术刊物,如Advanced Science, ACS Catalysis, Chemical Science, Chem, Chemical Reviews, Green Chemistry, Organic Letters等上公开发表论文近百篇,其中以第一作者或通讯作者身份公开发表84篇论文,包含ESI热点论文1篇,ESI高被引论文4篇,一篇入选2022 Green Chemistry Hot Articles论文,一篇论文曾被ACS Catalysis主编选为ACS Editors' Choice,H-index 28,多篇论文被RSC、Frontiers Journals、Organic Chemistry Portal、X-MOL、CBG、化学加、科研云等作为亮点进行报道和评述。
主持国家自然科学基金项目、江苏省自然科学基金项目、甘肃省科技基金项目等5项,参与撰写书章节1章,已申请或授权的专利三十多项。目前为Journal of the American Chemical Society, Angewandte Chemie International Edition, Fundamental Research等期刊审稿人,教育部学位中心评审专家,南京市科技局项目评审专家,江苏省对外科学技术促进会会员,曾获江苏省科协青年会员(化学化工领域)创新创业大赛一等奖。2023-2024年连续入选全球前2%顶尖科学家年度榜单(World's Top 2% Scientists)。
教育、工作经历:
5)2019.01-至今,南京工业大学,化学与分子工程学院,讲师、副教授
4)2022.12-2023.12,麦吉尔大学,博士后(合作导师:李朝军教授,加拿大)
3)2017.07-2018.12,南京工业大学,先进化学制造研究院,助理教授(合作者:沈志良教授;罗德平教授,新加坡)
2)2012.09-2017.06,苏州大学,博士,有机化学(导师:纪顺俊教授和徐小平研究员)
1)2008.09-2012.06,苏州大学,学士,化学(导师:纪顺俊教授和徐小平研究员)
研究方向:
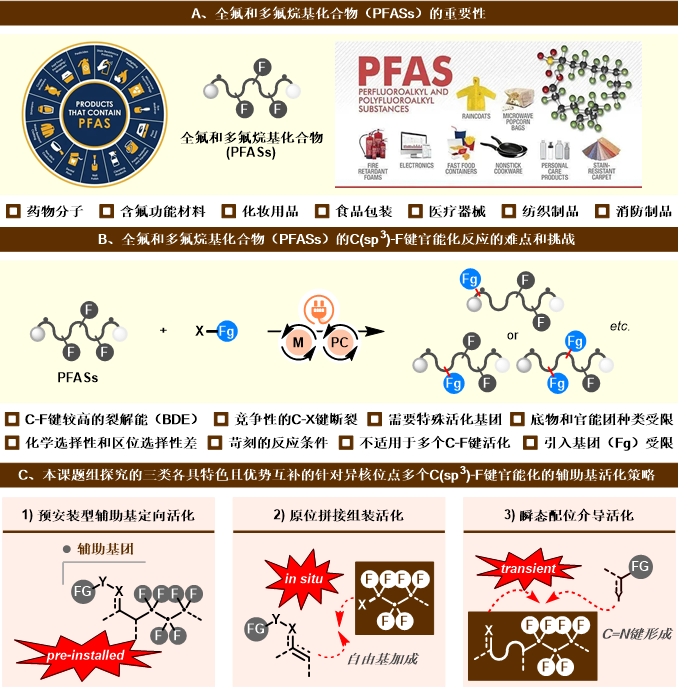
多氟和全氟烷基化合物(PFASs)转化、绿色化学
科研项目:
国家自然科学基金-青年(No. 22001121),负责人,结题;
甘肃省科技基金-青年(No. 25JRRF004),负责人,在研;
江苏省自然科学基金-青年(No. BK20180690),负责人,结题;
南京工业大学科研启动经费(No. 39837146),负责人,结题;
江苏省高校重点实验室(智能医用传感材料与器件)科研培育经费,负责人,在研;
国家自然科学基金-面上基金(No. 21772138),参与,结题;
所获荣誉:
教育部国家奖学金(2011 & 2015 & 2016);
苏州大学各类奖学金(2010-2017);
教育部《中国研究生》杂志123期封面人物;
“德国林岛诺贝尔获得者大会”评选活动,苏州大学代表(2017);
苏州“四创”好青年候选人;
苏州大学优秀博士论文(2017);
苏州大学优秀博士毕业生(2017);
国家留学基金委资助(2019);
南京工业大学优秀本科论文指导教师(2020 & 2022 & 2023);
南京工业大学化学与分子工程学院教育教学贡献奖(2021 & 2022);
南京工业大学化学与分子工程学院科学研究贡献奖(2021 & 2022);
南京工业大学化学与分子工程学院青年教师授课竞赛二等奖(2022);
南京工业大学微课竞赛二等奖(2022);
第七届江苏省科协青年会员(化学化工领域)创新创业大赛一等奖(2022);
第八届江苏省科协青年会员(化学化工领域)创新创业大赛三等奖x2(2023);
第九届江苏省科协青年会员(化学化工领域)创新创业大赛一等奖、三等奖x4(2024);
南京工业大学第十八届本科生学术科技论坛优秀指导教师三等奖(2023);
南京工业大学2023校级教改课题(20230102);
入选2023 & 2024全球前2%顶尖科学家年度榜单(World's Top 2% Scientists) (https://jokergoooo.shinyapps.io/top2pct_scientists/);
教学和学生培养信息:
主讲《有机化学C》、《有机化学实验》、《物理化学C》、《物理化学实验》
指导本科生(张要威、李琳、刘丽丽)获第十四届本科生学术科技论坛三等奖;
指导本科生获南京工业大学优秀本科毕业论文(2020届郭一聪;2022届王明铭;2023届罗鑫龙;2025届张鹏园);
指导本科生(郭一聪)获得2020届江苏省优秀本科毕业论文三等奖;
指导研究生获南京工业大学优秀研究生学位论文(2020届谢婷;2023届孙莉雯、于子伦;2024届陈玉兰、那金赫);
指导本科生主持国家级(罗鑫龙:202110291019Z;张鹏圆:202310291048Z;嵇一凡:2025)、省级(郑人浚)和多项校级(张要威、王茂林)大学生创新创业训练计划项目;
指导/协助指导研究生获国家奖学金(2018:员金金;2019:程步清;2020:宋轩笛;2021:张斯旋;2022:孙莉雯、陈玉兰、于子伦、李文欣、马娜娜、郭檬檬;2023:陈玉兰、陈佳伟、那金赫、任静奥、胡亚菲;2024:季文俊、冯曼航、胡轩搏、覃干棋、韩晓伟、韩薇、胡亚菲、陈雪、霍波洁);
江苏省研究生科研与实践创新计划项目(2023:那金赫:KYCX23_1403、陈玉兰:KYCX23_1401;2025:黄雪莹:KYCX25_1702、张驰:KYCX25_1703)
指导研究生(孙莉雯)获2022年化学与分子工程学院“长青杯”研究生科技论坛暨校第二十四届研究生科技论坛分论坛一等奖;
指导研究生(孙莉雯)获2022南京工业大学第二十四届科技论坛校二等奖;
南京工业大学化学与分子工程学院教改课题-一般项目(2022YB01);
指导本科生(罗鑫龙)获南京工业大学第十八届本科生学术科技论坛优秀作品奖特等奖;
指导本科生(张鹏圆)获“药石杯”第二届南京工业大学化学实验创新设计大赛三等奖;
指导研究生(陈玉兰)获2023年化学与分子工程学院“长青杯”研究生科技论坛暨校第二十五届研究生科技论坛分论坛二等奖;
指导本科生(张鹏圆)获第十九届本科生学术科技论坛二等奖;
指导研究生(陈玉兰)获2023南京工业大学“瑞华杯”大学生年度人物提名奖-学术科研类
指导研究生(陈玉兰)获2024年江苏省“优秀毕业生”;
指导研究生(韩薇)获2024年“长青杯”研究生科技论坛暨校第二十六届研究生科技论坛分论坛一等奖;
指导研究生(胡亚菲)获2024年“长青杯”研究生科技论坛暨校第二十六届研究生科技论坛分论坛三等奖;
指导研究生(韩薇)获2024年南京工业大学第二十六届科技论坛校二等奖;
指导研究生(胡亚菲)获2024年南京工业大学“瑞华杯”大学生年度人物提名奖-学术科研类
指导研究生(张驰、唐铭遥、高书籍)获2025年“长青杯”研究生科技论坛暨校第二十七届研究生科技论坛分论坛三等奖x3;
指导本科生(嵇一凡、陈丁鹏)获“药石杯”2025年第三届南京工业大学化学实验创新设计大赛暨第五届全国大学生化学实验创新设计大赛校内选拔赛决赛二等奖x2;
代表性论文(5篇):
5. Y.-L. Chen, W. Han, Y.-Y. Ren, M. Ma, D. Ge, Z.-L. Shen,* K. Guo,* X.-Q. Chu(褚雪强),* Defluorinative Cyclization of Enamides with Fluoroalkyl Halides through Two Vicinal C(sp3)-F Bonds Functionalization, Adv. Sci. 2025, 12, 202404738. (IF 2025 = ; Collections: Hot Topic: Fluorine Chemistry; highlighted by化学深耕堂; 有机那点事; X-Mol; Chin. Chem. Lett.)
4. J.-W. Chen, W.-J. Ji, X.-Y. Huang, D. Ge, Z.-L. Shen,* K. Guo,* X.-Q. Chu(褚雪强),* Chemo-, Regio-, and Stereoselective Tetrafunctionalization of Fluoroalkynes Enables Divergent Synthesis of 5–7-Membered Azacycles, Chem. Sci. 2024, 15, 12026−12035. (IF 2024 = 7.6; Most popular 2024 organic chemistry articles; Chemical Science 2024 最受欢迎文章合辑; highlighted by CBG)
3. X.-Q. Chu(褚雪强), R.-F. Cheng, C.-J. Li,* Palladium-Catalyzed Alkylation and Dienylation of Propargylic Carbonates with Hydrazones through Carbonyl Umpolung, ACS Catal. 2024, 14, 574−584. (IF 2023 = 12.9; highlighted by 化学深耕堂, CBG)
2. X.-Q. Chu(褚雪强),* D. Ge, Y.-Y. Cui, Z.-L. Shen,* C.-J. Li.* Desulfonylation via Radical Process: Recent Developments in Synthetic Applications. Chem. Rev. 2021, 121, 12548–12680. (IF 2021 = 60.622; Invited Review; ESI Hot Articl; ESI Highly Cited Paper; highlighted by X-MOL)
1. X.-Q. Chu(褚雪强), D. Ge, Z.-L. Shen,* T.-P. Loh,* Recent Advances in Radical-Initiated C(sp3)-H Bond Oxidative Functionalization of Alkyl Nitriles. ACS Catal. 2018, 8, 258−271 (IF = 13.084; Review; ACS Editors' Choice; ESI Highly Cited Paper).
