Intermolecular Reductive Heck Reaction of Unactivated Aliphatic Alkenes with Organohalides
Kewang Zheng,a Guanlin Xiao,a Tao Guo,a Yalan Ding,a Chengdong Wang,a Teck-Peng Loh,ab* and Xiaojin Wu a*
a Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China
b Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637616 (Singapore)
Abstract: A general intermolecular reductive Heck reaction of organohalides with both terminal and internal unactivated aliphatic alkenes has been first realized in high yield with complete anti-Markovnikov selectivity. The challenging vinyl bromides, aryl chlorides, and polysubstituted internal alkenes were first applied. More than 100 remote carbofunctionalized alkyl carboxylic acid derivatives were rapidly synthesized from easily accessible starting materials. The synthesis of drug molecules has further demonstrated the wide synthetic utility of this scalable strategy. Preliminary mechanistic studies are consistent with the proposed catalytic cycle.
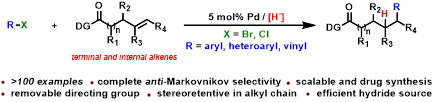
Org. Lett. 2020, DOI: org/10.1021/acs.orglett.9b04474(影响因子:6.555)
文章链接:https://doi.org/10.1021/acs.orglett.9b04474
