Mechanistic insight into the Organocalcium-mediated Nucleophilic Alkylation of Benzene and Further Rational Design
Xuefei Zhao, Dengmengfei Xiao, Xianlu Cui, Chaoqun Chai and Lili Zhao *
Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China.
Abstract:DFT calculations have been performed to study the recently reported unprecedented nucleophilic alkylation of benzene mediated by an organocalcium compound. The study reveals that the dimeric reaction mechanism is kinetically more favorable, and the rate-determining step is predicted to be the first nucleophilic attack of benzene by the organocalcium compound. Remarkably, the dimeric mechanism involves the formation of NMR detected dimeric alkyl calcium analogue 4, which agrees well with the experimental observations. EDA-NOCV analysis indicated that the origin of the higher reactivity of alkylcalcium 1 lies in its unusual bonding nature. We further studied the ligand effects and rationally designed the [(SiMe3BDI)CaEt]2 molecule, which shows a higher reactivity towards benzene. In addition, we de novo designed the more reactive heavier alkylstrontium and alkylbarium congeners, which might be targets for experimental synthesis.
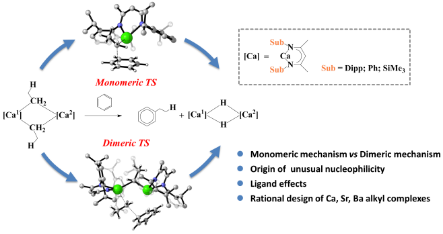
本论文封面以太极图为灵感,单金属中心机理和双金属中心机理对立又统一地阐明了苯的亲核取代反应机理。
Catalysis Science & Technology, 2020, 10, 950–958 (内封面文章,影响因子:5.726)
论文链接:https://pubs.rsc.org/en/content/articlelanding/2020/cy/c9cy02252j#!divAbstract
