Synthesis of Di(hetero)aryl Sulfides by Defluorinative Sulfenylation of Polyfluoroalkyl Ketones with Sodium Sulfinates or Arylsulfonyl Chlorides
Xue-Qiang Chu,*a Ting Xie,a Ya-Wen Wang,a Xiang-Rui Li,a Weidong Rao,b Haiyan Xu,c and Zhi-Liang Shen*a
a Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China.
b Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-Forest Biomass, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China.
c School of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang, Jiangsu 212003, China.
Abstract: A facile incorporation of privileged sulfide, naphthofuran framework, and perfluoroalkyl moiety in one molecule was successfully accomplished through tandem defluorinative sulfenylation of a-perfluoroalkyl ketones with sulfur source. The reaction presumably proceeds via a sequence involving defluorination, reductive sulfenylation, autoaromatization, and annulation, accompanied with the simultaneous cleavage of four C(sp3)-F bonds and the formation of new C-S and C-O bonds.
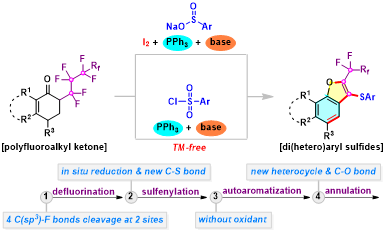
Chem. Commun. 2020, 56, 8699-8702. (Impact factor: 5.996).
论文链接:https://pubs.rsc.org/en/content/articlelanding/2020/cc/d0cc03303k
