Indium-mediated difunctionalization of iodoalkyl-tethered unactivated alkenes via an intramolecular cyclization and an ensuing palladium-catalyzed cross-coupling reaction with aryl halide
Xuan-Di Song,a Xiang-Rui Li,a Ya-Wen Wang,a Xue-Qiang Chu,a Weidong Rao,b Haiyan Xu,c Guo-Zhi Han*a and Zhi-Liang Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China
b Jiangsu Key Laboratory of Biomass-based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China
c School of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang, Jiangsu 212003, China
Abstract: An efficient cobalt-catalyzed, indium-mediated difunctionalization of iodoalkyl-tethered unactivated alkenes via cyclization/cross-coupling sequence was developed. The reactions proceeded effectively with wide functional group tolerance, leading to a wide variety of cyclic compounds, including cyclopentane, furan, pyrrolidine, octahydro-1H-indene, octahydro-benzofuran, hexahydro-4H-furo[2,3-b]pyran, and hexahydro-furo[2,3-b]furan, which are pivotal cores widely found in pharmaceutical and bioactive compounds.
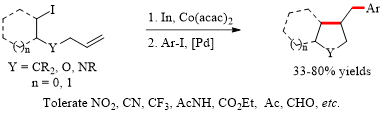
Organic Chemistry Frontiers 2020, DOI: 10.1039/D0QO00632g. (Impact factor: 5.155)
论文链接:https://pubs.rsc.org/en/Content/ArticleLanding/2020/QO/D0QO00632G#!divAbstract
