Three-Component Heteroannulation for Tetrasubstituted Furan Construction Enabled by Successive Defluorination and Dual Sulfonylation Relay
Song-Zhou Cai,a,d Danhua Ge,a,d Li-Wen Sun,a Weidong Rao,b Xin Wang,c Zhi-Liang Shen,*a and Xue-Qiang Chu*a
a Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: ias_zlshen@njtech.edu.cn; xueqiangchu@njtech.edu.cn.
b Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-Forest Biomass, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China.
c Hubei Province Geological Experimental Testing Center, Wuhan 430034, China.
d S.-Z. Cai and D. Ge contributed equally to this work.
Abstract: Synthetic strategies by making use of one-pot multi-step cascade reactions are of special interest. Herein, an efficient three-component tandem reaction of polyfluoroalkyl peroxides with sulfinates for the facile construction of fluoroalkylated tetrasubstituted furan derivatives has been developed. The combination of DABCO and Cs2CO3 was found to be essential for the success of the reaction. This modular and regioselective approach proceeded via an unprecedented sequence of successive defluorination, dual sulfonylation, and annulation relay, along with four C(sp3)-F bonds cleaved and two new C-S bonds formed. In addition, this transition metal-free C-F bond functionalization which is amenable to gram-scale synthesis occurred under mild reaction conditions and has broad substrate scope and excellent functional group tolerance. Moreover, this defluorinative protocol also enabled the late-stage functionalization of complex compounds, which could potentially find synthetic utility in drug discovery.
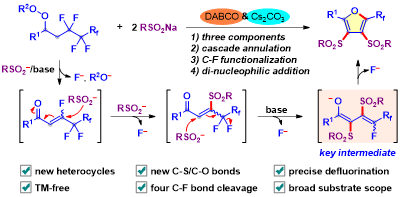
Green Chemistry 2021, 23, 935-94. (Impact factor: 9.480)
论文链接:https://pubs.rsc.org/en/content/articlelanding/2021/gc/d0gc03922e#!divAbstract
