Photoredox-Catalyzed Regio- & Stereoselective C(sp2)–H Cyanoalkylation of Enamides with Cycloketone Oximes via Selective C-C Bond Cleavage/Radical Addition Cascade
Ting Guan,†a,d Jing-Yu Guo,†a,d Qing-Hong Zhang,a Xin-Wen Xu,a Xiao-Yu Yu,a Yu Zhang,b and Kai Zhao*a,c
aInstitute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing 211816, China
bCollege of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China
cGuangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen 518055, China
dPharmaBlock Sciences (Nanjing), Inc, 10 Xuefu Road, Jiangbei New Area, Nanjing, 210032, China
Abstract: A photoredox-catalyzed regio- and stereoselective Heck-type cyanoalkylation of synthetically prominent enamides with cycloketone oximes via selective β-C-C bond scission/selective radical addition cascade is developed, enabling the incorporation of synthetically versatile and pharmaceutically appealing distal cyanoalkyl moieties into enamide scaffolds under mild conditions. The synthetic importance of this methodology was highlighted by the broad substrate scopes, satisfying functional group compatibilities, excellent regio- and stereoselectivities as well as the versatile and diverse synthetic applications of β-cyanoalkylated enamides.
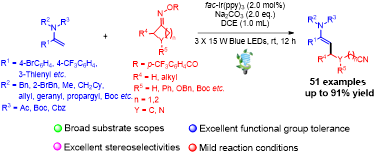
Green Chemistry. 2022, 24, 6524–6530. (2021年影响因子:11.034).
论文链接:https://doi.org/10.1039/D2GC01978G
