Benzylic Aroylation of Toluenes Mediated by a LiN(SiMe3)2/Cs+ System
Yuanyun Gu, Zhen Zhang, Yan-En Wang, Ziteng Dai, Yaqi Yuan, Dan Xiong, Jie Li, Patrick J. Walsh,* and Jianyou Mao
aTechnical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu
Road, Nanjing 211816, P.R. China
Abstract: Chemoselective deprotonative functionalization of benzylicC−H bonds is challenging, because the arene ring contains multiple aromatic C(sp 2 )−H bonds, which can be competitively deprotonated and lead to selectivity issues. Recently it was found that bimetallic [MN(SiMe3) 2 M = Li, Na]/Cs+ combinations exhibit excellent benzylic selectivity. Herein, is reported the first deprotonative addition of toluenes to Weinreb amides mediated by LiN(SiMe3)2/CsF for the synthesis of a diverse array of 2-arylacetophenones. Surprisingly, simple methyl benzoates also react with toluenes under similar conditions to form 2-arylacetophenones without double addition to give tertiary alcohol products. This finding greatly increases the practicality and impact of this chemistry. Some challenging substrates with respect to benzylic deprotonations, such as fluoro and methoxy substituted toluenes, are selectively transformed to 2-aryl acetophenones. The value of benzylic deprotonation of 3-fluorotoluene is demonstrated by the synthesis of a key intermediate in the preparation of Polmacoxib.
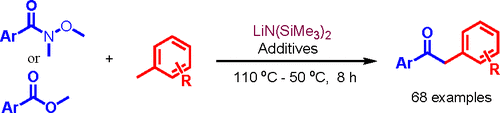
J. Org. Chem/ 2022, 87, 1, 406–418 (2021年影响因子4.198)
论文链接:https://pubs.acs.org/doi/full/10.1021/acs.joc.1c02446
