Palladium-catalyzed enantioselective (2-naphthyl)methylation of azaarylmethyl amines
Shuguang Chen, Jiahong Tan, Dan Xiong, Yongjia Shang, Jianyou Mao*a and Patrick J. Walsh
aTechnical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu
Road, Nanjing 211816, P.R. China
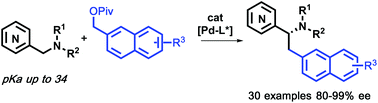
Abstract: Enantioenriched azaarylmethyl amine derivatives are useful building blocks in synthetic and medicinal chemistry. To access these valuable motifs, an enantioselective palladium-catalyzed benzylation of azaarylmethyl amine pronucleophiles is introduced. Of note, this is a rare application of asymmetric (2-naphthyl)methylation of pro-nucleophiles with high pKa values (pKa≈34 in DMSO). Control experiments support the notion that the coordination of Li+ to the azaaryl nitrogen plays a critical role in the substitution process. With this procedure, enantioenriched (2-naphthyl)methylene azaarylmethyl amines with a variety of azaaryl groups (pyridyl, pyrazine, quinoxaline and isoquinoline) and cyclic and acyclic amines are readily obtained with good yields and enantioselectivities up to 99%.
Organic Chemistry Frontiers (2022), 9(10), 2721-2727 (2022年影响因子5.456)
论文链接:https://pubs.rsc.org/en/content/articlelanding/2022/qo/d2qo00273f
