DMSO-Promoted Difluoroalkylation of Organophosphonium Salts with Difluoroenol Silyl Ethers
Z.-L. Yu,#a J.-W. Chen,#a Y.-L. Chen,a R.-J. Zheng,a M. Ma,b J.-P. Chen,*a Z.-L. Shen,*a X.-Q. Chu*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China.
b Department of Chemistry and Materials Science, College of Science, Nanjing Forestry University, Nanjing, Jiangsu 210037, China.
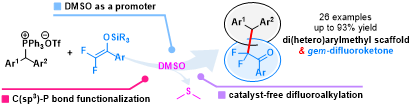
Abstract: An efficient method for the synthesis of β,β-di(hetero)aryl-α,α-difluorinated ketones using readily available organophosphonium salts and difluoroenol silyl ethers has been developed. This mild reaction features a good functional group tolerance, a scaled-up synthesis, and synthetic simplicity. By taking advantage of DMSO as a less-toxic promoter and solvent for the difluoroalkylation and C−P bond functionalization, the use of transition-metal catalysts and sensitive additives could be avoided.
Org. Lett. 2022, 24, 5557–5561, DOI: 10.1021/acs.orglett.2c02088. (Impact factor: 6.072, #Equal contribution, highlighted by CBG)
论文链接:https://pubs.acs.org/doi/10.1021/acs.orglett.2c02088
