Reductive-Delay Heck 1,1-Diarylation of Terminal Alkenes
Huihui Shao,† Yao Zhao,† Shuangqiang Wang,‡ Rizhi Chen,‡ Jianrong Steve Zhou,§ and Xiaojin Wu†*
† Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China
‡ State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 211816, China
§ State Key Laboratory of Chemical Oncogenomics, Guangdong Provincial Key Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen 518055, China
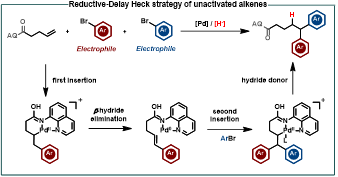
Abstract: Pd-catalyzed chemo- and regiocontrollable 1,1-diarylation of unactivated aliphatic alkenes with two aryl halides was developed. Under the cationic reductive-delay Heck pathway, the first aryl insertion is followed by β-H elimination, while the second aryl insertion is terminated by C−H bond formation.
Org. Lett. 2022. DOI: 10.1021/acs.orglett.2c02416. (Impact factor: 6.072)
论文链接: https://doi.org/10.1021/acs.orglett.2c02416
