Photocatalyzed Borylcyclopropanation of Alkenes with a
(Diborylmethyl)iodide Reagent
Jiefeng Hu,*[a,b] Man Tang,[a] Jing Wang,[a] Zhu Wu,[b] Alexandra Friedrich,[b] and Todd B. Marder*[b]
[a] School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing, 211816 Jiangsu (China)
[b] Institut für Anorganische Chemie and Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg (Germany)
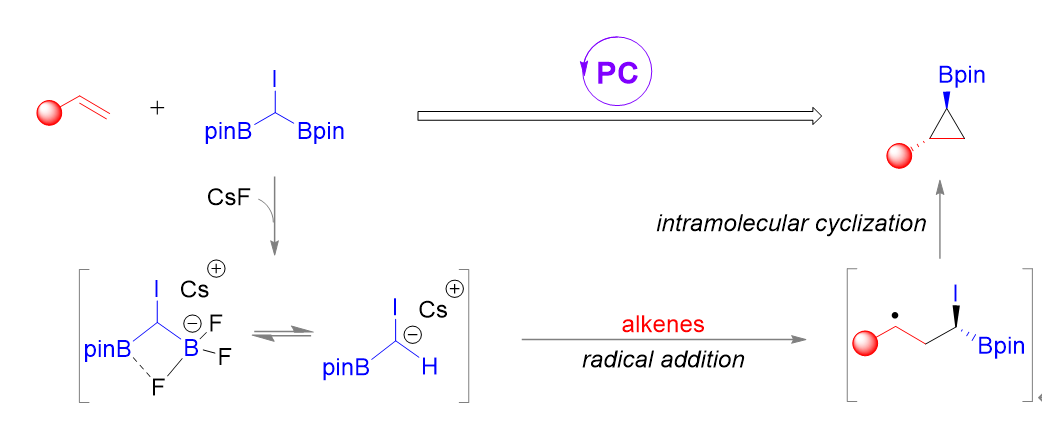
Abstract: Cyclopropane skeletons play a prominent role in the development of organic synthesis and pharmaceutical chemistry. Herein, we report the design and synthesis of a stable, multifunctional (diborylmethyl)iodide reagent (CHI(Bpin)2) for the photoinduced cyclopropanation of alkenes, providing an array of 1,2-substituted cyclopropylboronates in good yields. This α-haloboronic ester can be readily synthesized on a multigram scale from commercially available starting materials. Furthermore, the protocol displays high chemo- and diastereoselectivity, excellent functional-group tolerance, and allows for late-stage borylcyclopropanation of complex molecules. Mechanistic studies reveal that the borylcyclopropanation proceeds through a radical addition/polar cyclization pathway mediated by the photocatalyst fac-Ir(ppy)3 and visible light.
Angew. Chem. Int. Ed. 2023, e202305175. (影响因子: 16.6).
论文链接:https://doi.org/10.1002/anie.202305175
