“On-Water” Synthesis of Sterically Congested γ-Ketophosphine Oxides Featuring a CF3- and P(O)-Tetrasubstituted Carbon Center
Man-Hang Feng,a Wen-Jun Ji,a Bo-Jie Huo,a Hao Xu,a* Mengtao Ma,b Zhi-Liang Shen,a* and Xue-Qiang Chua*
a Technical Institute of Fluorochemistry, Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, People’s Republic of China. E-mail: xuhao@njtech.edu.cn; ias_zlshen@njtech.edu.cn; xueqiangchu@njtech.edu.cn.
b Department of Chemistry and Materials Science, College of Science, Nanjing Forestry University, Nanjing 210037, People’s Republic of China.
Abstract: The distinctive hydrophobic hydration effect in the aqueous solution contributes to the success of the phospha-Michael reaction between β-CF3-β,β-disubstituted enones and diarylphosphine oxides, providing an access to biologically relevant γ-ketophosphine oxides bearing a sterically highly congested CF3- and P(O)-tetrasubstituted carbon center. More importantly, the resulting products could be further transformed into densely functionalized γ-ketophosphine oxides containing a CF3- and P(O)-tetrasubstituted carbon center.
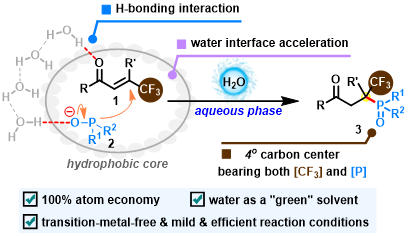
Advanced Synthesis & Catalysis 2023, 365, 3546-3552. (Impact factor: 5.4)
论文链接:https://onlinelibrary.wiley.com/doi/abs/10.1002/adsc.202300741
