Nickel-catalyzed cross-electrophile coupling of aryl thiols with aryl bromides via C–S bond activation
Hao Xu,*a Cai-Yu He,a Bo-Jie Huo,a Jia-Wen Jing,a Chengping Miao,b Weidong Rao,c Xue-Qiang Chu,a Xiaocong Zhou,*b and Zhi-Liang Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: xuhao@njtech.edu.cn; ias_zlshen@njtech.edu.cn.
b College of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China. E-mail: xczhou@zjxu.edu.cn.
c Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-Forest Biomass, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China.
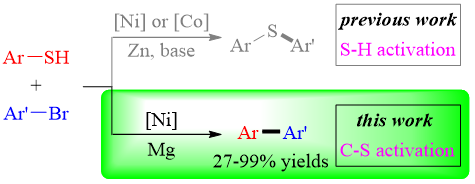
Abstract: The reaction of aryl thiols with aryl bromides usually provides thioethers as the major products, even in the presence of a transition metal catalyst and a zinc mediator. We report here a cross-electrophile coupling of aryl thiols with aryl bromides via C–S bond activation instead of S–H bond cleavage. The reaction proceeded effectively in the presence of a nickel catalyst, magnesium turnings, and lithium chloride in THF at room temperature to afford a variety of structurally diverse biaryls in moderate to good yields.
Organic Chemistry Frontiers 2023, 10, 5171-5179. (Impact factor: 5.4)
论文链接:https://pubs.rsc.org/en/content/articlelanding/2023/qo/d3qo01236k
