“On-water” defluorophosphorylation of trifluoromethylated enones with phosphine oxides
Xue-Qiang Chu,*a Li-Wen Sun,a Cheng Ma,d Jia-Wei Chen,a Yu-Lan Chen,a Shao-Fei Ni,*d Ming-Quan Zhu,a Jie Zhou,*b Mengtao Mac and Zhi-Liang Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: xueqiangchu@njtech.edu.cn; ias_zlshen@njtech.edu.cn.
b State Key Laboratory of Materials-Oriented Chemical Engineering, College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: jayzhou@njtech.edu.cn.
c Department of Chemistry and Materials Science, College of Science, Nanjing Forestry University, Nanjing 210037, China.
d Department of Chemistry, Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, 515063, China. E-mail: sfni@stu.edu.cn.
Abstract: The distinctive fluorine effects on “on-water” chemistry have remained less explored. In this work, an “on water” reaction of β-trifluoromethylated enones with phosphine oxides was developed for the preparation of highly functionalized gem-difluorodienes with excellent Z-selectivity. The reaction occurred through a well-designed tandem phospha-Brook rearrangement and defluorination sequence under transition-metal-free conditions in water solution, which efficiently overcame some competitive side reactions, such as hydrodefluorination, 1,4-nucleophilic addition, and phosphorylation. The success was attributed to favorable H-bonding interactions at the water–organic phase boundary which could activate the substrates and enhance the reaction selectivity.
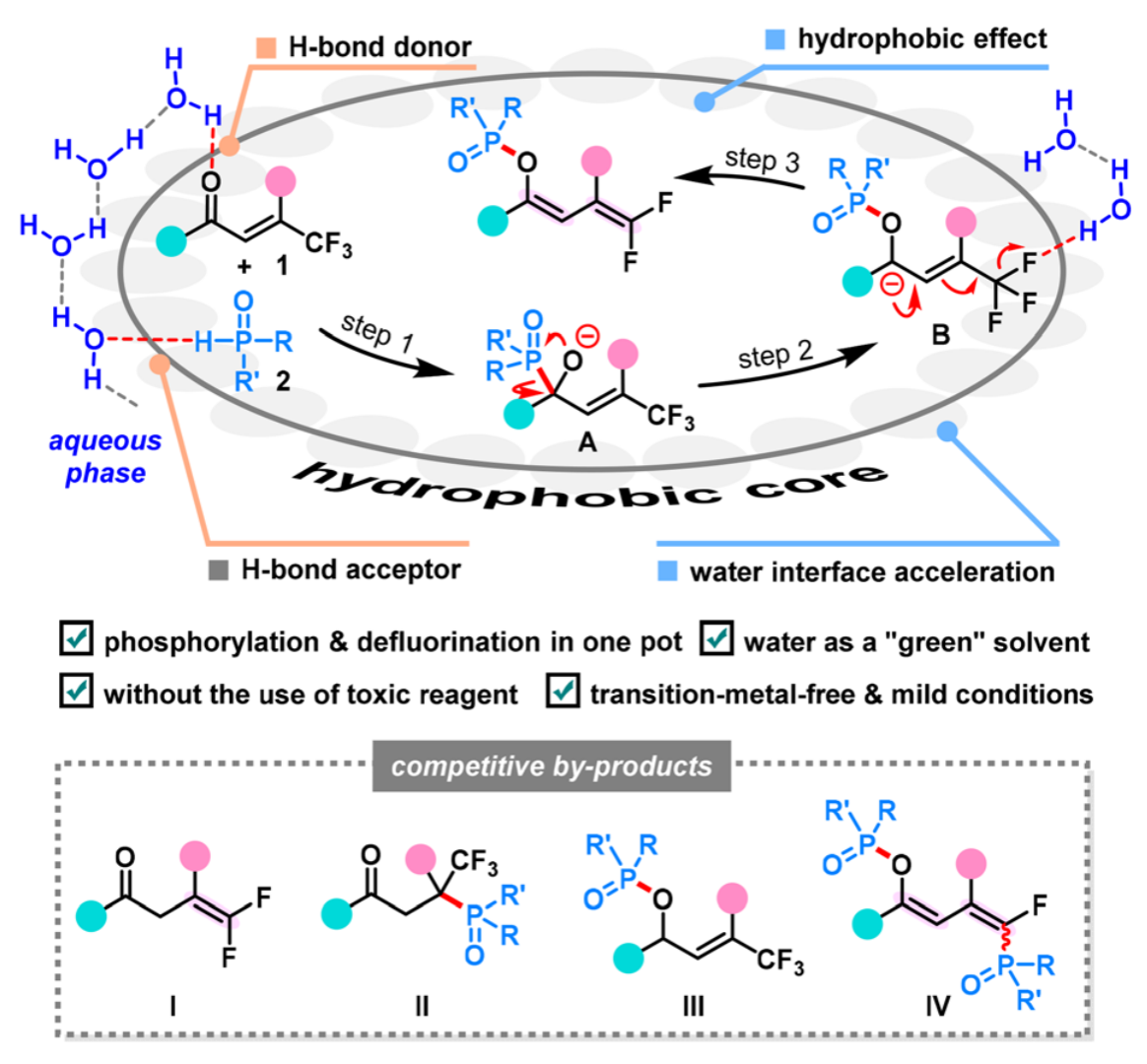
Green Chemistry 2023, 25, 6489-6497. (Impact factor: 9.8)
论文链接:https://pubs.rsc.org/en/content/articlelanding/2023/GC/D3GC01565C
