Transition-metal-catalyzed asymmetric defluorinative reactions
Danhua Ge,*a Jia-Wei Chen,a Yu-Lan Chen,a Mengtao Ma,b Zhi-Liang Shen*a and Xue-Qiang Chu*a
a Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: ias_zlshen@njtech.edu.cn; xueqiangchu@njtech.edu.cn.
b Department of Chemistry and Materials Science, College of Science, Nanjing Forestry University, Nanjing 210037, China.
Abstract: Organofluorides have found wide applications in materials science, medicinal chemistry, agrochemistry, and organic synthesis. The unique reactivity and properties of fluorine-containing compounds render them worth investigating in asymmetric synthesis to access valuable enantioenriched molecules. Although metal catalysis paves the way for the chemoselective and regioselective defluorination of fluorine-containing molecules, the functionalization of the elusive C–F bond integrated into catalytic enantioselective transformation remains highly sought after. In this review, the latest achievements in transition-metal-catalyzed enantioselective defluorinative coupling reactions have been comprehensively summarized on the basis of the classification of transition-metal catalysts. Compared with other heteroatoms, special emphasis is placed on the function of the fluorine atom as a detachable “chemical handle” for the enantioselective transformation of easily accessible fluorine-containing chemicals, such as trifluoromethyl alkenes, allylic fluorides, propargyl gem-difluorides, gem-difluoroalkenes, polyfluoroarenes, dienyl fluorides, and trifluoromethyl (hetero)arenes. We aim to inspire chemists to continue their interest in both research topics of C–F bond activation and asymmetric catalysis.
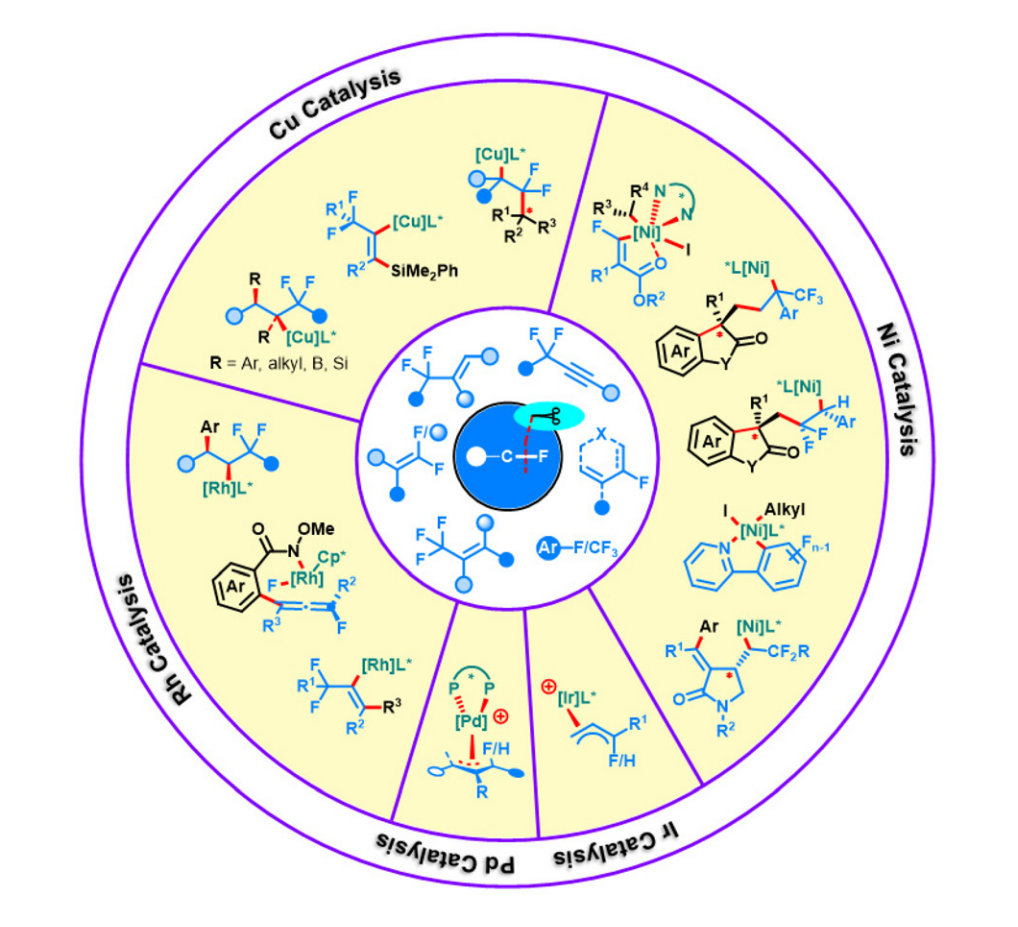
Organic Chemistry Frontiers 2023, 10, 3909-3928. (2023 Organic Chemistry Frontiers HOT articles. Impact factor: 5.4)
论文链接:https://pubs.rsc.org/en/content/articlelanding/2023/QO/D3QO00751K
