Na2S·9H2O Enabled Defluorodisulfuration and Hydrodefluorination
of Perfluorobutyl Tetralones: Synthesis of Trifluoromethyl 1,2-
Dithioles
Zi-Lun Yu,a Man-Hang Feng,a Peng-Yuan Zhang,a Hao Xu,a Danhua Ge,a Mengtao Ma,b Zhi-Liang Shen,*,a
and Xue-Qiang Chu*,a
a Technical Institute of Fluorochemistry, Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: ias_zlshen@njtech.edu.cn; xueqiangchu@njtech.edu.cn.
b Department of Chemistry and Materials Science, College of Science, Nanjing Forestry University, Nanjing 210037, China.
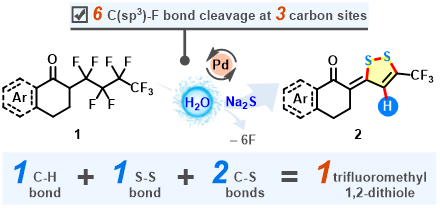
Abstract: An unprecedented defluorocyclization of perfluorobutyl tetralones with Na2S‧9H2O was developed for the synthesis of tri-fluoromethyl 1,2-dithioles, which provided chemists novel access to biologically and pharmaceutically relevant organofluo-rides. Successive C(sp3)-F bond functionalization at the perfluoroalkyl chain is vital for four C-H/C-S/S-S bonds formation and a five-membered S-heterocycle assembly. Cheap, low-toxic, and odorless inorganic sulfide Na2S‧9H2O acts as both a disulfurating precursor and a hydrodefluorinating reagent in this tandem multi-bond-interconverting reaction.
Organic Letters 2023, 25, 4388-4393. (Impact factor: 5.2)
论文链接:https://pubs.acs.org/doi/10.1021/acs.orglett.3c01573
