Nickel-catalyzed direct cross-coupling of aryl fluorosulfates with aryl bromides
Jin-He Na,a Xiang Liu,a Jia-Wen Jing,a Jing Wang,b Xue-Qiang Chu,a Mengtao Ma,c Hao Xu,*a Xiaocong Zhou,*b and Zhi-Liang Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: xuhao@njtech.edu.cn; ias_zlshen@njtech.edu.cn.
b College of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China. E-mail: xczhou@zjxu.edu.cn.
c College of Science, Nanjing Forestry University, Nanjing 210037, China.
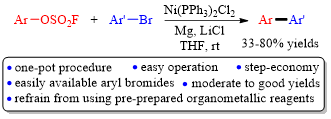
Abstract: A one-pot, direct cross-coupling of aryl fluorosulfate with aryl bromide, which is step-economical and avoids the use of preprepared/commercial organometallic reagent, could be accomplished by performing the reaction in THF at room temperature in the presence of nickel catalyst, magnesium turnings, and lithium chloride, giving rise to the corresponding biaryls in moderate to good yields with reasonable functional group compatibility.
Organic Letters 2023, 25, 2318-2322. (Impact factor: 5.2)
论文链接:https://pubs.acs.org/doi/10.1021/acs.orglett.3c00674
