Nickel-Catalyzed Direct Cross-Coupling of Aryl Thioether with Aryl Bromide
Na-Na Ma,a Xuan-Bo Hu,a Yuan-Shuai Wu,a Ya-Wen Zheng,a Mengtao Ma,b Xue-Qiang Chu,a Hao Xu,*a Haiqing Luo,*c and Zhi-Liang Shen*a
a Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: xuhao@njtech.edu.cn; ias_zlshen@njtech.edu.cn.
b College of Science, Nanjing Forestry University, Nanjing 210037, China.
c Department of Chemistry & Chemical Engineering, Gannan Normal University, Ganzhou 341000, China E-mail: luohaiq@sina.com.
Abstract: A straightforward cross-coupling of aryl thioether with aryl bromide with the aid of nickel salt, magnesium, and lithium chloride in tetrahydrofuran at ambient temperature was accomplished. The one-pot reactions proceeded efficiently via C−S bond cleavage to produce the desired biaryls in modest to good yields, avoiding the use of pregenerated or commercial organometallic reagents.
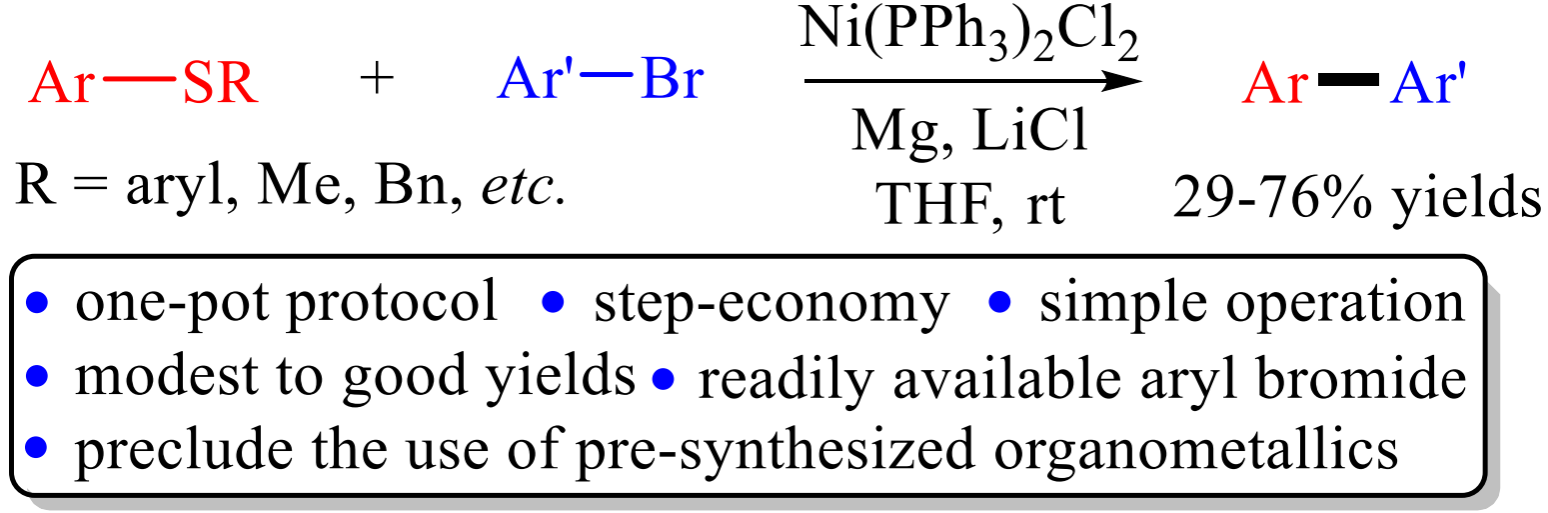
Organic Letters 2023, 25, 1771-1775. (Impact factor: 5.2)
论文链接:https://pubs.acs.org/doi/10.1021/acs.orglett.3c00518
