Synergistic Copper-Aminocatalysis for Direct Tertiary α-Alkylation of Ketones with Electron-Deficient Alkanes
Qi-Chao Shan1,§, You-Wei Wu1,§, Mu-Xiang Chen1, Xuefei Zhao1, Teck-Peng Loh2,3,*, and Xu-Hong Hu1,*
1 Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China
2 College of Advanced Interdisciplinary Science and Technology, Henan University of Technology, 100 Lianhua Street, Zhengzhou 450001, China
3 Division of Chemistry and Biological Chemistry, School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Singapore
§Q.-C.S. and Y.-W.W. contributed equally to this work.
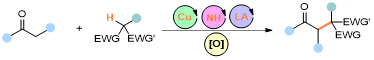
Abstract: In this study, we present a novel approach for the tertiary α-alkylation of ketones using alkanes with electron-deficient C−H bonds, employing a synergistic catalytic system combining inexpensive copper salts with aminocatalysis. This methodology addresses the limitations of traditional alkylation methods, such as the need for strong metallic bases, regioselectivity issues, and the risk of over alkylation, by providing a high reactivity and chemoselectivity without the necessity for pre-functionalized substrates. The dual catalytic strategy enables the direct functionalization of C(sp3)−H bonds, demonstrating remarkable selectivity in the presence of conventional C(sp3)−H bonds that are adjacent to heteroatoms or π systems, which are typically susceptible to single-electron transfer processes. Our findings contribute to the advancement of alkylation techniques, offering a practical and efficient route for the construction of C(sp3)−C(sp3) bonds, and paving the way for further developments in the synthesis of complex organic molecules.
Adv. Sci. 2024, 11, 2402255 (影响因子: 15.1)
论文链接:https://onlinelibrary.wiley.com/doi/10.1002/advs.202402255
